Chemistry 11
Homogeneous and Heterogeneous
Introduction to chemistry
- Chemistry is the study of properties of matter and the changes that matter undergoes.
- Hypothesis is an educated guess about how things work, is the prediction of the outcome of the experiment results.
- By making sure that only one factor is changed at a time, while keeping all other conditions the same, you can ensure that an experiment is a "fair test".
- Steps of the scientific method
- ask a question
- make observations
- form a hypothesis
- conduct an experiment
- analyze data
- draw a conclusion
- Significant Figures and Scientific Notations.
- WHMIS - Workplace Hazardous Material Information System
- MSDS - Material Safety Data Sheets, one of 4 main components of WHMIS.
Matter
- Pure substances
- Elements are homoatomic substances that cannot be decomposed into simpler materials by chemical reactions
- Compounds are heteroatomic substances formed from two or more elements in which the elements are combined in the same fixed proportion
- Mixtures
- solution (e.g. salt water)
- mechanical mixture (Sulphur and lead mixed together)
Atomic theory
- Three fundamental laws of chemistry
- Conservation of Mass - the total mass of all reactants will equal the total mass of products
- Definite proportions - a specific compound always contains the same elements in the same definite proportions
- Multiple proportions - some combinations of elements may be capable of forming more than one compound
- Pd (lead), Au (Gold), Hg (Mercury), Sn (Tin), Zn (Zinc)
Periodic table
- Group 1 - Alkali metals
- Group 2 - Alkaline earth metals
- Group 17 - Halogens
- I Br-ing Cl-ay F-rom O-ur N-ew H-ouse.
- Group 18 - Noble gases
- Trends
- Titanium is more metallic than Iron, since Ti is located right to the iron at the same period on the periodic table. Since the metallic character decreased from the left to the right across a period, so Titanium is more metallic.
- Fluorine is located to the left of Carbon at the same period. As the electronegativity increases from left to right across a period, so Fluorine has a higher electronegativity than Carbon.
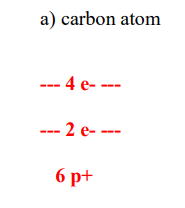
Bohr and Quantum Theory
- Electron configuration
- Cu -
- Co using core notation -
- Cu -
- Bohr diagram
- Lewis diagram
- Valence electrons refers to the total electrons in the outermost incomplete energy level of an atom
- Structural diagrams (no electrons)
Science review
- Certain groups on the periodic table have combining capacities (charges) that need memorization.
- Chlorine is in Group 17, the combining capacity is -1
(Beryllium Oxide) (Barium Phophide) (Silicon Tetroxide), (Trinitrogen monoxide), (Triphosphorus Octoxide) (Nickel (III) Oxide) - the Roman Numeral to use is determined by calculation, need to check the combining capacity. (Iron (II) Sulphide)
- Gold (I) Hypochlorite - Strontium phosphate - Bismuth (V) cyanide - Calcium Sulphite
(Hydrosulfuric acid), (Hydrophosphoric acid) (Sulfuric acid), (Phosphoric acid) (Sulfurous acid), (Nitrous acid)
- [[Ions_Periodic_Table.pdf]] via Science Geek
Mole
- one mole is the number of atoms in exactly 12 grams of Carbon 12. The value of a mole is
, noted using n. - Avagadro's Number:
- Number of particles N.
- Molar mass of
= 87.91 g/mole.
Molar volume of Gases
- STP: Standard Temperature is
and Pressure and the standard pressure if 101.3 kPa. Molar volume of gases is 22.4 L/mol - SATP: Standard Ambient Temperature at
and Pressure of 100 kPa respectively. The volume of one mole of gas at SATP is 24.8 L/mol.
Molar concentration (Molarity)
- Solute is the substance dissolved in a solvent to form a solution, while solvent is the substance that performs the dissolving in a solution.
- Molarity unit: M = mol/L
Solutions and Dilutions
- Volumetric flask has etched mark on its neck indicating the accurate total volume of the solution, which has been calibrated by its manufacturer, while the graduations on a beaker are not accurate, only for crude approximations.
Empirical formula
- The percent composition of each element (in a compound) is the mass of the element divided by the molar mass times 100%, used to find the percentage of elements in a compound.
- The 3 steps to find the empirical formula of a compound are as follows
- assume 100g of the compound and convert percent compositions to grams
- Convert grams to each element to moles
- find the simplest whole number ratio among the moles, usually by dividing by the smallest number of moles.
- like the empirical formula of Benzene is
instead of
- The molecular formula is the chemical formula that indicates the numbers of each atom in a molecular compound whereas the empirical formula is the formula that gives the simplest whole number ratio of atoms of elements in a compound.
- The molecular formula will be determined by calculating based on a given molar mass
Chemical reactions
- 3 things conserved in a chemical reactions are
- the total number of each kind of atom
- the total mass - Law of the Conservation of Mass
- the total energy
- phase symbols (s), (l), (g), (aq).
- Balance the equations: easier to do the element found in the most compounds last and to start with the element that is simplest to balance.
- 6 types of reactions
- synthesis reaction
- decomposition reaction
- single replacement reaction -
- double replacement reaction -
- combustion reaction -
- neutralization reaction -
- Exothermic reactions - metabolizing food,
- Endothermic reactions - boiling water;
- Stoichiometry (the relationship between the relative quantities of substances taking part in a reaction or forming a compound, typically a ratio of whole integers).
- Limiting reagent is the reactant that runs out or is totally consumed after the reaction is complete.
- Excess reagent is the reactant that remain after the reaction is complete
- Percent yield is a measure of how much product was actually obtained in an experiment compare to how much was predicted by the stoichiometry calculation to be made, equals to
Solution chemistry
- pure substances
- has constant physical properties, like color, odor, and density
- has constant chemical properties, like reactivity, toxicity, and kindling temperature
- can be described by a formula
- mixtures
- has variable physical properties
- has variable chemical properties
- can be described only by a recipe
- e.g. a liquid dissolved in a solid is known as an amalgam, while a solid dissolved in another solid is know as alloy
- 4 factors affecting solubility are
- molecular polarity
- size of molecules
- temperature
- pressure
- Molar concentration
- long form of the concentration formula:
- the short form is C = n/V
- long form of the concentration formula:
- Electronegativity is the attraction that an atom has for a covalently bonded electron pair
- The symmetrical shape of
molecule dictates that each Cl is in direct competition with each other and there's no winner and no skewing of the electrons and their negative charges. Thus, the molecule as a whole is non-polar. - non-polar solute dissolves in non-polar solvent
- polar solute dissolves in polar solvent
- ionic solute dissolves in polar solvent
- molecular compounds have covalent bonds, which are formed by shared electrons between atoms. When these dissolve, they usually separate into individual but whole neutral molecules, instead of dissociating into ions.
- Covalent solutions, or molecular solutions, do not form charged particles (ions). So, there's nothing to conduct electrons through the solution, thus they are unable to conduct electricity.
- positive ions are cations
- negative ions are anions
Organic chemistry
- a carbon atom can form 4 covalent bonds
- 4 most common elements found in organic compounds
- oxygen, carbon, hydrogen, nitrogen, phosphorus
- The first organic compound created from purely inorganic compounds are urea,
- Alkanes: hydrocarbons in which the carbon atoms are singly bonded to each other.
- general formula is
- -ane to name all saturated hydrocarbons: methane, ethane, propane, butane, pentane, hexane, heptane, octane, nonane, decane
- general formula is
- alkenes are considered unsaturated because the carbons involved in the double bonds are holding fewer hydrogens than they would be if the bond was a single bond.
- general formula is
.
- general formula is
- alkynes - general formula
. - triple bond is the strongest and most react out of the 3 types of bonds.
- 3 ways to represent organic compounds
- a molecular formula
- a structural diagram (formula)
- a condensed structural formula
- substitution reactions is where a halogen takes the place of a hydrogen in an alkane
- The molecular formula is the same for structural isomers, while the structural formula would be different for structural isomers.
Naming organic compounds
- find the longest carbon train
- -yl is used to name substituent alkyl groups.
- -ol is used to name alcohol (ethanol)
Hydrocarbon derivatives and synthesis
- Aldehyde, -al
- ketone, -one
- ether, longer side -ane, shorter side -oxy
- carboxylic acid, -oic acid
- ester side without C=O is -yl, side with C=O is -oate.
- synthetic fibers are made from polypropylene (addition polymers).
- Different from the formation of an ester, the formation of a condensation polymer involves the polymerization process which happens at both ends of both molecules.